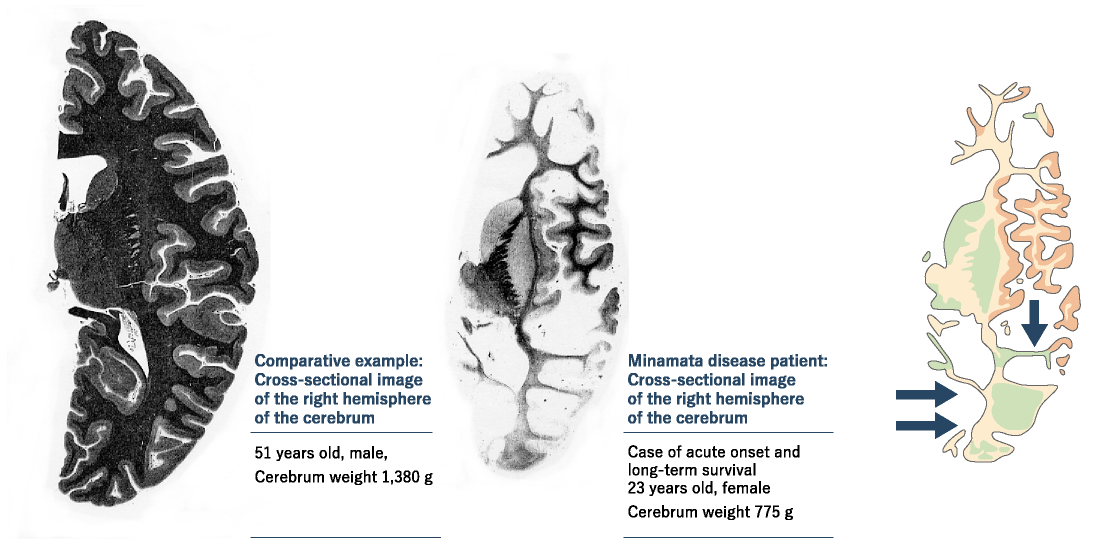
Research on mercury
Variety of Mercury
Studies on Mercury
Few scientists had been interested in mercury until methylmercury was found to be a cause of Minamata disease.
Research in natural science, such as medicine on mercury has been greatly developed after outbreak of Minamata disease.
However, there still remain a lot of subjects to be clarified on how mercury compounds including methylmercury function inside the human body.
Variety of Mercury
There are various types of mercury compounds (inorganic mercury and organic mercury). You can find below a few examples:
|
Metallic mercury
(Hg0) 
|
The most familiar mercury used in thermometer. Inhalation of its vapor causes damage to lung, kidney and brain. |
|---|---|
|
Mercuric oxide
(HgO) 
|
Almost insoluble in water. Used as a topical antiseptic. Mercuric sulfide. |
|
Mercuric sulphide
(HgS) 
|
Insoluble in water. Used as a red pigment in ancient times. Most mercury in natural environment exists as this form. |
|
Mercuric chloride
(HgCl2) 
|
Often used in toxicology research. Given to experimental animals by injection due to poor absorption from the gastric tube. Causes severe renal failure. |
|
Methylmercuric chloride
(CH3HgCl) 
|
Cause of Minamata Disease. On absorption, it is widely found tissues. |
Recycle of mercury
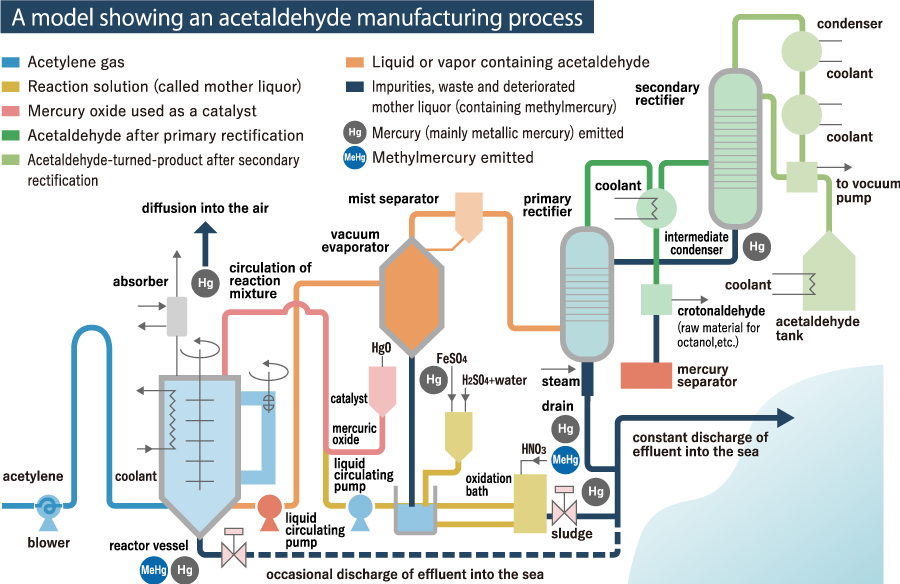
Mercury is widely used in the commodities such as battery cells for watch and camera, fluorescent light tubes, liquid crystal display screens of cellular phone and computers. When commodities containing mercury are out of use, they are disposed as wastes. Recycling of mercury is required in order to prevent environmental pollution from happening and re-use resources effectively by recovering them from wastes.
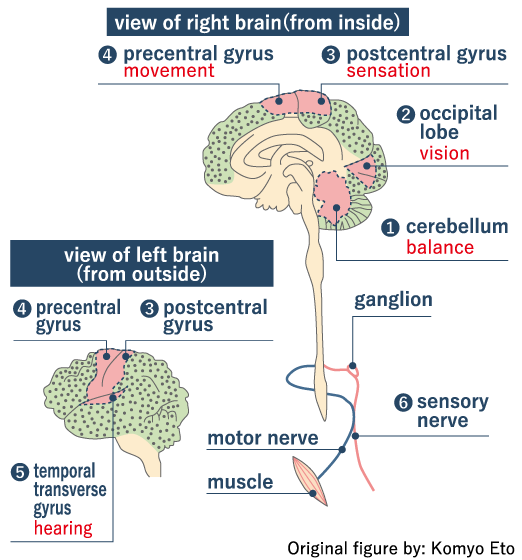
Fate of Mercury in the Environment
Fate of Mercury in the Environment
Mercury, having come out onto the land surface as a result of volcanic activities or the use of fossil fuels, circulates in nature as it changes its shape. For the most part, it comes in the form of a safe compound called mercuric sulfide. On the other hand, mercury sometimes - rarely but sometimes - changes into methylmercury, which can enter into our body through a food chain containing aquatic creatures. Its impact, however, remains very small and normally involves no health damage. It poses a problem when, for some reason, a high density of mercury pollution occurs in a localized fashion.
Circulation of mercury
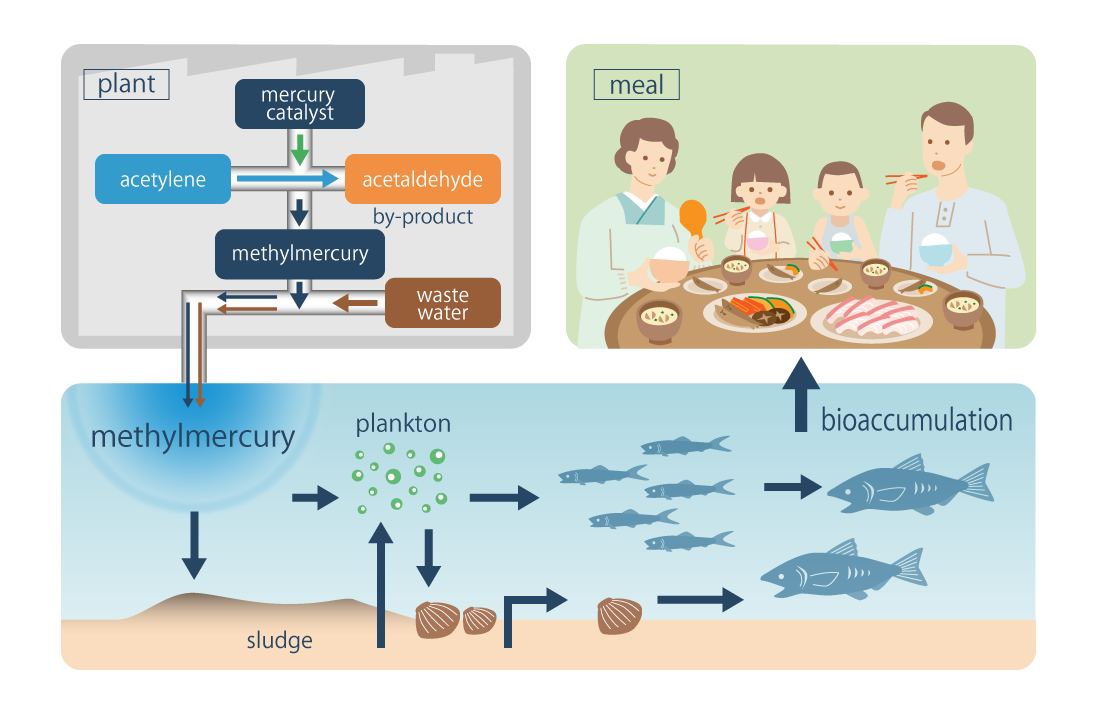
An Illustration of Mercury Bioaccumulation by Food Chain
Methylmercury in sea water is taken into aquatic animals through gill or intestine directly. On the other hand, the concentration of mercury tends to be higher as the trophic ranking be higher. It is called bio-accumulation.
The figure shows relation between trophic ranking mercury level of sedentary fishes in Minamata Bay when mercury-polluted sludge still remained in the bay.
Mercury concentration of the sedentary fishes in Minamata Bay in 1985
Development in Mercury Analysis
Measuring methods: Past and present
Past(before 1960)
Colorimetry (analysis of total mercury)
Total mercury can be analyzed by density of orange color of mercury-dithizone complex.
About 1/1,000,000 g (1 microgram) of mercury is detected by this method.

dithizone-mercury complex
Present (NIMD Method)
Atomic absorption method(analysis of total mercury)
Mercury vapor originated from the mercury-containing samples can be detected by its specific ultraviolet absorption.
To vaporize mercury (mostly in a form of mercuric compound) in the samples, (i) reductive method and (ii) combustion method are employed.
Less than 1/1,000,000,000 g (1 nano gram) of mercury can be detected by this method.
Apparatus for total mercury analysis
-

Reductive volatilizing apparatus
Stannous ion (Sn2+) reduces mercuric ion (Hg2+) to mercury vapor (HgO) -

Heating at 800℃ for several minutes
Changes mercuric ion (Hg2+) to mercury vapor(HgO)
ECG-GC method(analysis of methylmercury)
Methylmercury in the sample solution must be extracted into organic solvent such as toluene prior to analysis.Methylmercury chloride in the toluene solution can be detected by ECG-GC (Electron-Capture Detector-Gas Chromatography).
Less than 1/10,000,000,000 g (0.1 nano gram) of mercury can be detected by this method
Apparatus for methylmercury analysis
-

ECD-GC
Recovery of Mercury from Mercury-Polluted Soil by Thermal Treatment
Low temperature thermal treatment system for mercury-polluted soil.
This system has been developing for remediation with removing the mercury from mercury-polluted soil/sediment. We succeeded in removing mercury from mercury-polluted soil and sediment by low temperature thermal treatment with using particular additives. Further efforts are has been made with the aim of practical technology.
1. Threw polluted soil/sediment into the apparatus and mix it with additive in it.
2. As soil/sediment is heated to 300℃ by rotary kiln, the mercury vaporizes from mercury-polluted soil/sediment.
3. Completion of treatment. Treated soil/sediment is clean.
4. This devise removes dust in the exhaust gas.
5. Recovering mercury vaporized with thermal treatment by scrubber.
6. Emit the clean exhaust gas into the air.